The Alzheimer's Crisis: Understanding the Disease Burden
The Current State of Alzheimer's Treatment
Alzheimer's disease represents the sixth leading cause of death in the United States and affects approximately 6.7 million older Americans currently, with projections indicating this number will triple by 2050 absent disease-modifying interventions. Globally, nearly 40 million individuals suffer from Alzheimer's disease or related dementias, creating devastating impact on patients, families, and healthcare systems. Despite decades of research investment and pharmaceutical development, no disease-modifying treatments currently exist—available medications provide only temporary, modest symptomatic relief without halting disease progression.[1]
The prevailing scientific consensus, held for decades, stated that Alzheimer's disease represents an irreversible neurodegenerative condition: once cognitive decline begins, the damage cannot be repaired, and the best possible interventions merely slow disease progression toward inevitable dementia and death. This assumption profoundly influenced research priorities, therapeutic development, and patient counseling. If the condition is irreversible, treatment focuses on slowing progression rather than reversal.[1]
What is Alzheimer's Disease?
Why Reversal Was Considered Impossible
Multiple factors supported the belief that Alzheimer's represents an irreversible condition. Neuroimaging studies show progressive brain volume loss and neuronal death in Alzheimer's patients. Autopsy studies reveal extensive amyloid plaques and tau tangles—protein pathologies considered hallmarks of neuronal death. The assumption followed logically: once neurons die, they cannot be regenerated; therefore, once cognitive decline occurs, recovery appears impossible. This reasoning shaped decades of research focused on slowing amyloid and tau accumulation rather than attempting reversal.[1]
![]()
The Breakthrough: Reversing Alzheimer's Through NAD+ Restoration
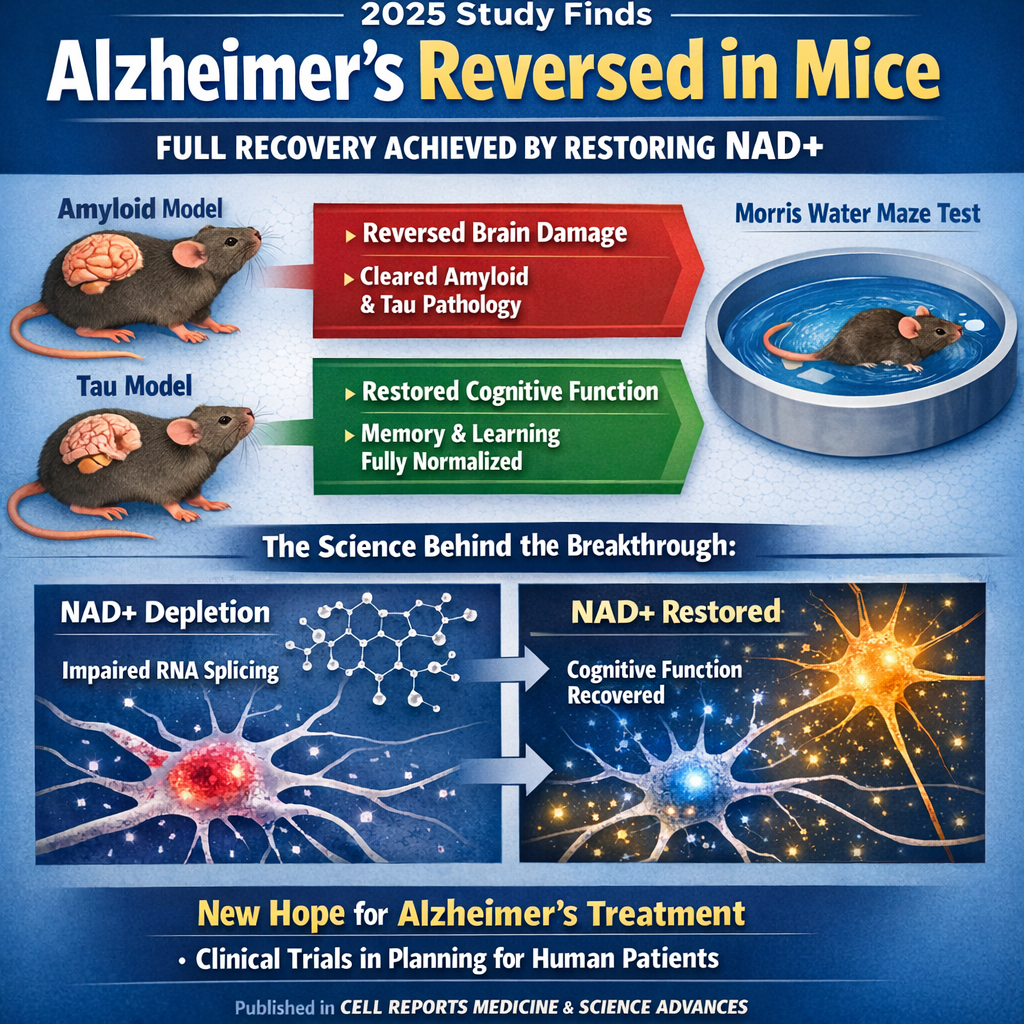
In 2025, Dr. Andrew A. Pieper and colleagues at Case Western Reserve University demonstrated something previously thought impossible: that advanced Alzheimer's disease can be completely reversed in mice, with full restoration of cognitive and memory function. Using two genetically distinct mouse models of Alzheimer's—one developing amyloid plaques, the other tau tangles—researchers administered P7C3-A20, an experimental compound that restores cellular NAD+ balance.[1]
The results exceeded the most optimistic expectations: mice with advanced Alzheimer's-like pathology and significant cognitive impairment were treated and subsequently performed identically to healthy, age-matched control mice on multiple measures of memory and cognitive function. The treatment achieved what had never before been demonstrated: complete functional recovery in an advanced Alzheimer's disease model.[1]
Remarkable Behavioral Recovery
In the Morris water maze—a gold-standard test of spatial memory and cognitive function where mice must find a hidden platform in a pool of water—untreated Alzheimer's mice struggled significantly, taking substantially longer to locate the platform and showing poor memory retention on subsequent days. Treated mice, by contrast, performed identically to healthy control mice, finding the platform in normal timeframes and demonstrating normal memory retention.[1]
Similar improvements appeared across multiple cognitive tests: object recognition tests showed treated Alzheimer's mice performed identically to healthy controls, remembering previously encountered objects. Fear-conditioning tests showed normal learning and memory. All measures of cognitive function normalized completely with P7C3-A20 treatment.[1]
Spatial Learning & Memory (Morris Water Maze)
![]()
The Mechanism: NAD+ and Brain Energy Balance
What Is NAD+? The Energy Molecule Critical to Life
NAD+ (nicotinamide adenine dinucleotide) represents a crucial coenzyme present in virtually every cell, serving multiple critical functions in cellular energy production, DNA repair, and cellular stress responses. NAD+ is essential for mitochondrial function—the energy-producing organelles within cells—and for sirtuins, proteins regulating cellular aging, inflammation, and stress resilience. Without adequate NAD+, cells struggle to produce energy, mount stress responses, and repair damage.[1]
As individuals age, NAD+ levels naturally decline—a process accelerated dramatically in Alzheimer's disease. This NAD+ depletion represents a fundamental problem underlying Alzheimer's pathology: without sufficient NAD+ for energy production and stress response, neurons become dysfunctional and vulnerable to damage.[1]
The NAD+ Depletion Hypothesis of Alzheimer's Disease
The new research advances a paradigm-shifting hypothesis: Alzheimer's disease pathology may not primarily result from irreversible neuronal death but rather from reversible neuronal dysfunction caused by impaired NAD+ homeostasis and brain energy failure. When NAD+ levels are insufficient, neurons cannot maintain proper function including normal protein folding, protein trafficking, synaptic transmission, and neuronal communication—functions that can be restored if NAD+ balance is restored.[1]
This hypothesis explains multiple Alzheimer's features: amyloid and tau accumulation, neuroinflammation, synaptic dysfunction, and cognitive decline all result from energy failure and impaired cellular stress responses. When NAD+ is restored, enabling normal neuronal energy metabolism, these dysfunctions resolve and cognition recovers.[1]
P7C3-A20: The Compound That Restores NAD+ Balance
Rather than directly delivering NAD+ (which cannot cross the blood-brain barrier effectively), P7C3-A20 works through an elegant mechanism: it enhances cells' intrinsic capacity to restore NAD+ balance when under stress. Think of NAD+ depletion as a "leaky bucket" losing energy; P7C3-A20 doesn't pour more water in—rather, it fixes the leak.[1]
The compound works by enhancing NAD+ salvage pathways—the cellular mechanisms recycling NAD+ and replenishing depleted pools. Cells treated with P7C3-A20 restore depleted NAD+ to normal levels, enabling restoration of normal energy metabolism, stress responses, and neuronal function.[1]
ZEISS Microscopy for Neuroscience Research
Amyloid-PET imaging in Alzheimer's disease - Neurotorium
![]()
Molecular Mechanisms: The NAD+-EVA1C Splicing Axis
Alternative RNA Splicing and Neuronal Function
Recent 2025 research published in Science Advances uncovered the specific molecular mechanism through which NAD+ depletion causes Alzheimer's pathology and how NAD+ restoration reverses it. The mechanism involves alternative splicing—the process where genes can be "read" in multiple ways, producing different protein variants with different functions.[1]
Dysfunctional alternative splicing represents a hallmark of aging and Alzheimer's disease, with many genes producing aberrant protein variants in Alzheimer's brains. This splicing dysfunction contributes to neuronal dysfunction and cognitive decline. The research demonstrated that NAD+ augmentation corrects these abnormal splicing patterns by regulating a key protein called EVA1C (epithelial V-like antigen 1 homolog C).[1]
EVA1C: The Critical Recovery Protein
EVA1C emerged as central to NAD+-mediated Alzheimer's recovery: EVA1C levels are reduced in Alzheimer's patient brains compared to cognitively normal individuals, and NAD+-induced memory improvement was partially dependent on EVA1C. Genetic knockdown of Eva1c (reducing its expression) substantially impaired NAD+-induced memory recovery in tau-mutant mice, demonstrating that EVA1C is necessary for NAD+ therapeutic effects.[1]
This discovery suggests that NAD+ protects the brain through restoring proper EVA1C function, which in turn corrects aberrant RNA splicing patterns characteristic of Alzheimer's disease. The NAD+-EVA1C splicing axis represents a fundamental neuronal resilience pathway, maintaining proper gene expression patterns essential for neuronal function and memory.[1]
Convergence of Multiple Protective Mechanisms
Beyond splicing correction, NAD+ restoration activates multiple protective mechanisms simultaneously:[1]
· Enhanced mitochondrial function: Restoring NAD+ enables normal ATP (energy) production in mitochondria, providing neurons with energy needed for synaptic transmission and function
· Improved DNA repair: NAD+ is essential for DNA repair enzyme function; restoration enables repair of accumulated DNA damage in Alzheimer's brains
· Reduced neuroinflammation: NAD+ restoration reduces excessive activation of brain immune cells (microglia and astrocytes), normalizing inflammatory responses
· Enhanced neuronal stress responses: NAD+-dependent pathways including sirtuins become functional, enabling cells to mount proper stress responses
· Improved synaptic plasticity: NAD+ restoration enhances long-term potentiation (LTP)—the synaptic strengthening underlying learning and memory
![]()
The Two-Mouse Model Validation: Different Pathologies, Similar Recovery
Amyloid and Tau Models Show Convergent Recovery
A critical strength of the research involved demonstrating complete recovery in two genetically distinct mouse models, each developing different Alzheimer's pathologies:[1]
Model 1: Amyloid Pathology Model: Mice with genetic mutations causing amyloid-beta accumulation, forming amyloid plaques in the brain similar to human Alzheimer's disease
Model 2: Tau Pathology Model: Mice with genetic mutations causing tau protein abnormalities and tau tangle formation, mimicking tau pathology in human Alzheimer's
Both models developed cognitive impairment similar to human Alzheimer's disease. Both showed complete cognitive recovery with P7C3-A20 treatment. This convergence demonstrates that the therapeutic mechanism—NAD+ restoration—addresses a fundamental pathophysiological process operative in both amyloid and tau-driven Alzheimer's forms.[1]
Implications for Human Alzheimer's Disease
The fact that NAD+ restoration reverses both amyloid and tau-driven disease strongly suggests that impaired brain energy metabolism represents a fundamental upstream pathophysiological mechanism in Alzheimer's disease, present across different genetic forms and disease variants. This convergence suggests that NAD+ restoration might benefit diverse Alzheimer's patient populations, regardless of which specific pathological hallmarks predominate.[1]
![]()
Biomarker Changes: Evidence of True Pathological Reversal
Normalization of Phosphorylated Tau (p-tau217)
Beyond behavioral recovery, researchers measured biochemical and pathological markers of Alzheimer's disease and found that treatment normalized these markers. P-tau217 (phosphorylated tau at position 217), an established biomarker used in human Alzheimer's diagnosis and clinical trials, was elevated in untreated Alzheimer's mice and normalized to healthy control levels with P7C3-A20 treatment.[1]
This normalization of p-tau217 is particularly significant: in clinical trials, p-tau217 levels predict cognitive decline risk and serve as validated markers of disease modification in human Alzheimer's patients. The fact that P7C3-A20 normalized this human-relevant biomarker suggests the treatment targets fundamental disease mechanisms relevant to human disease.[1]
Protein Profile Changes Matching Human Alzheimer's Recovery
Researchers compared protein expression changes in treated mice with databases of human Alzheimer's brain tissue (from autopsy studies). They identified 46 proteins that were abnormally expressed in both human Alzheimer's brains and untreated Alzheimer's mice. Remarkably, P7C3-A20 treatment normalized all 46 proteins to normal levels. These proteins control cellular stress responses, energy production, and inflammation—suggesting that NAD+ restoration corrects fundamental pathophysiological processes disrupted in human Alzheimer's disease.[1]
![]()
Translational Potential: From Mouse Model to Human Clinical Trials
P7C3-A20: Safety and Tolerability Profile
Before pursuing human clinical trials, researchers evaluated P7C3-A20's safety in nonhuman primates (macaque monkeys)—a crucial step in translating experimental compounds to human treatment. Daily oral administration for 38 weeks caused no toxicity, no adverse effects, and was well-tolerated, with consistent plasma levels and no induction of metabolic clearance pathways. This exceptional safety profile significantly increases the likelihood of successful human trials.[1]
Clinical Trial Planning and Timeline
Following the dramatic 2025 mouse study results, Dr. Pieper emphasized that human clinical trials represent the critical next step. Clinical trials are actively being planned to determine whether NAD+ restoration produces similar cognitive and pathological recovery in human Alzheimer's patients as observed in mice.[1]
Dr. Pieper stated: "This is important when considering patient care, and clinicians should consider the possibility that therapeutic strategies aimed at restoring brain energy balance might offer a path to disease recovery... This new therapeutic approach to recovery needs to be moved into carefully designed human clinical trials to determine whether the efficacy seen in animal models translates to human patients."[1]
Virtual Morris Water Maze - Maze Engineers
![]()
Understanding the Implications: From "Irreversible" to "Reversible"
Paradigm Shift in Alzheimer's Understanding
This research fundamentally challenges the concept of Alzheimer's as an irreversible, invariably progressive condition. If cognitive decline in Alzheimer's primarily reflects reversible neuronal dysfunction from energy failure rather than irreversible neuronal death, this opens entirely new therapeutic possibilities. The goal shifts from "slowing decline" to "restoring function."[1]
This paradigm shift has profound implications: if Alzheimer's can be reversed, patients could potentially recover lost cognitive abilities. This contrasts dramatically with current expectations where cognitive loss is universally considered permanent. The psychological and societal implications are staggering—from patients losing hope about their future to family members watching their loved ones recover lost abilities.[1]
Addressing the "Death vs. Dysfunction" Question
A critical question underlying these findings: are neurons in Alzheimer's disease actually dead, or are they dysfunctional? The complete cognitive recovery in mice suggests that many neurons survive despite appearing "damaged" on pathological examination—they're dysfunctional due to energy failure but retain capacity to recover. When energy metabolism is restored through NAD+ augmentation, these neurons resume normal function and cognition recovers.[1]
This distinction explains a long-standing puzzle: some Alzheimer's patients retain substantial neuronal populations despite severe cognitive symptoms, while autopsy studies show amyloid plaques and tau tangles in cognitively normal elderly individuals. This dissociation between pathology and symptoms suggests that pathological hallmarks alone do not determine function—energy status and neuronal resilience matter equally.[1]
![]()
Future Research Directions and Complementary Approaches
Identifying Essential Aspects of Brain Energy Balance
Dr. Pieper emphasizes additional research questions requiring investigation: "Additional next steps for the laboratory research include pinpointing which aspects of brain energy balance are most important for recovery, identifying and evaluating complementary approaches to Alzheimer's reversal, and investigating whether this recovery approach is also effective in other forms of chronic, age-related neurodegenerative disease."[1]
Broader Applications Beyond Alzheimer's
The NAD+ restoration approach may extend therapeutic benefits beyond Alzheimer's disease. Other age-related neurodegenerative diseases including Parkinson's disease, Huntington's disease, and age-related cognitive decline all involve impaired brain energy metabolism and declining NAD+ levels. NAD+ restoration might provide therapeutic benefits across multiple neurodegenerative conditions sharing energy metabolism dysfunction.[1]
Combination Therapies and Complementary Approaches
Future research may involve combining NAD+ restoration with complementary approaches targeting different pathophysiological mechanisms: anti-inflammatory treatments targeting neuroinflammation, amyloid-targeting approaches preventing further amyloid accumulation, tau-targeting therapies preventing tau propagation, and neuroprotective strategies supporting neuronal survival. Combination approaches may produce superior outcomes compared to monotherapy alone.[1]
![]()
Realistic Expectations: Translating Mouse Success to Human Treatment
The Mouse-to-Human Translation Challenge
While mouse study results represent genuine breakthroughs, translating these findings to effective human treatments requires careful assessment of realistic expectations. Mice and humans differ substantially: mice have different brain size, lifespan, genetic background, environmental exposures, and disease progression trajectories. A therapy producing complete recovery in mice may produce modest improvements in humans, or may not work at all—this is the fundamental challenge in translational neuroscience.[1]
Why This Research Is Still Genuinely Promising
Despite translation uncertainties, this research represents genuine breakthrough for multiple reasons:[1]
1. Multiple Proof Points: Complete recovery in two distinct disease models suggests the mechanism is robust and operates across diverse Alzheimer's forms
2. Human Biomarker Validation: The identified 46 proteins that normalize with treatment in mice similarly abnormal in human Alzheimer's brains, suggesting mechanism relevance to humans
3. Nonhuman Primate Safety: Safety data in primates supports likelihood that safety in mice translates to human safety
4. Mechanistic Understanding: The specific molecular mechanisms identified (NAD+-EVA1C splicing axis) provide rational basis for targeted human therapeutic development
5. Urgent Clinical Need: Current Alzheimer's treatments fail most patients; even modest improvements over current standard of care would represent meaningful progress
Timeline Expectations for Human Treatment Availability
If human clinical trials begin soon and proceed favorably, NAD+-restoring treatments might become available to patients within 5-10 years, though conservative estimates suggest 10-15 years before wide clinical availability. This timeline reflects standard phases of clinical trial development, regulatory approval, manufacturing scale-up, and clinical practice integration.[1]
![]()
Conclusion: A New Era in Alzheimer's Treatment
The 2025 demonstration that advanced Alzheimer's disease can be completely reversed in mice—achieving full restoration of cognitive and memory function through NAD+ restoration—represents a genuinely transformative breakthrough in neuroscience and geriatric medicine. This achievement directly challenges decades-old assumptions about Alzheimer's irreversibility and opens entirely new therapeutic possibilities.[1]
The underlying mechanism—that Alzheimer's pathology reflects reversible neuronal dysfunction from impaired brain energy metabolism rather than irreversible neuronal death—provides rational basis for targeted therapeutic interventions. The identification of the NAD+-EVA1C splicing axis reveals a fundamental neuronal resilience pathway that, when supported, enables cognitive recovery even in advanced disease.[1]
While translating mouse research to effective human treatments requires careful validation through clinical trials, the convergence of complete functional recovery in two distinct disease models, normalization of human-relevant disease biomarkers, and mechanistic understanding of the therapeutic pathway creates unprecedented optimism that NAD+-restoring treatments will provide genuine benefit to Alzheimer's patients.[1]
For the millions of patients and families devastated by Alzheimer's disease, this research offers something previously impossible to envision: genuine hope that cognitive loss might not be permanent, that lost memories might be recovered, that the inexorable progression to dementia and death might be reversible. The transition from "slowing Alzheimer's" to "reversing Alzheimer's" represents not merely an incremental therapeutic improvement but a paradigm shift in how medicine understands and treats one of humanity's most feared diseases. As clinical trials progress and human data emerge, Alzheimer's disease may finally transition from the category of untreatable conditions to treatable diseases offering genuine prospect of recovery and restored cognition.[1]
![]()
Citations:
Case Western Reserve University - New study shows Alzheimer's disease can be reversed (2025); Cell Reports Medicine - NAD+ reverses Alzheimer's neurological deficits via NAD+ restoration (2025); Science Advances - NAD+ reverses Alzheimer's neurological deficits via regulating differential alternative RNA splicing of EVA1C (2025); StudyFinds - Major Alzheimer's Breakthrough: Advanced-Stage Mice Fully Recover After Treatment (2025); PMC - NAD+ supplementation normalizes key Alzheimer's features and DNA damage responses (2018); PMC - NAD+ supplementation reduces neuroinflammation and cell senescence via cGAS-STING (2021); PMC - NAD+-boosting agent nicotinamide mononucleotide improves mitochondrial stress response (2024); PMC - Cognitive and Alzheimer's disease biomarker effects of oral nicotinamide riboside supplementation (2024); PMC - evoke and evoke+: Phase 3 studies evaluating semaglutide in early-stage symptomatic Alzheimer's disease (2025); PMC - Supplementation with NAD+ and Its Precursors to Prevent Cognitive Decline (2022); PMC - NAD+ in Alzheimer's Disease: Molecular Mechanisms and Systematic Therapeutic Evidence (2021); Nature Mental Health - Neuroprotective efficacy of P7C3 compounds in primate models (2018); Springer - Uncertainty in anti-amyloid monoclonal antibody therapy for Alzheimer's disease (2025); MDPI - Therapeutic Applications of Dental Mesenchymal Stem Cells in Alzheimer's Disease (2025); CARI Journals - Semaglutide's Role in Modulating the Brain-Heart Axis in Obesity and Alzheimer's (2025); Frontiers Neurology - Efficacy and safety of traditional Chinese medicine in Alzheimer's disease (2025); Alzheimer's & Dementia Journal - Alzheimer's Disease as Type 3 Diabetes: Semaglutide Potential (2025); IECC Mexico Journal - Neurodegeneration and Precision Medicine in Alzheimer's Therapy (2025); Medical Science Monitor - Real-World Outcomes of Disease-Modifying Therapies (2025); SAGE Journals - Comparative efficacy and safety of brexpiprazole doses for agitation in Alzheimer's (2025); Medical Xpress - NAD+ restores memory in Alzheimer's disease models (2025); NIA - NIA-Funded Active Alzheimer's and Related Dementias Clinical Trials (ongoing); ClinicalTrials.gov - NAD Augmentation to Prevent or Reverse Alzheimer's (NCT07278492)[1]





Post your opinion
No comments yet.