Genome editing using CRISPR/Cas9 to treat hereditary ...
Introduction: A Year of Unprecedented Medical Innovation
The year 2025 has proven to be a remarkable period for medical science, delivering breakthrough innovations that transition from theoretical possibility to clinical reality. While the pace of medical advancement has accelerated consistently over recent decades, 2025 stands apart for the sheer transformative potential of developments emerging across diverse domains—from fundamental molecular biology to clinical transplantation to pharmaceutical innovation. Each of these breakthroughs carries implications extending far beyond immediate clinical applications, suggesting that healthcare in the coming years will look fundamentally different from contemporary practice.
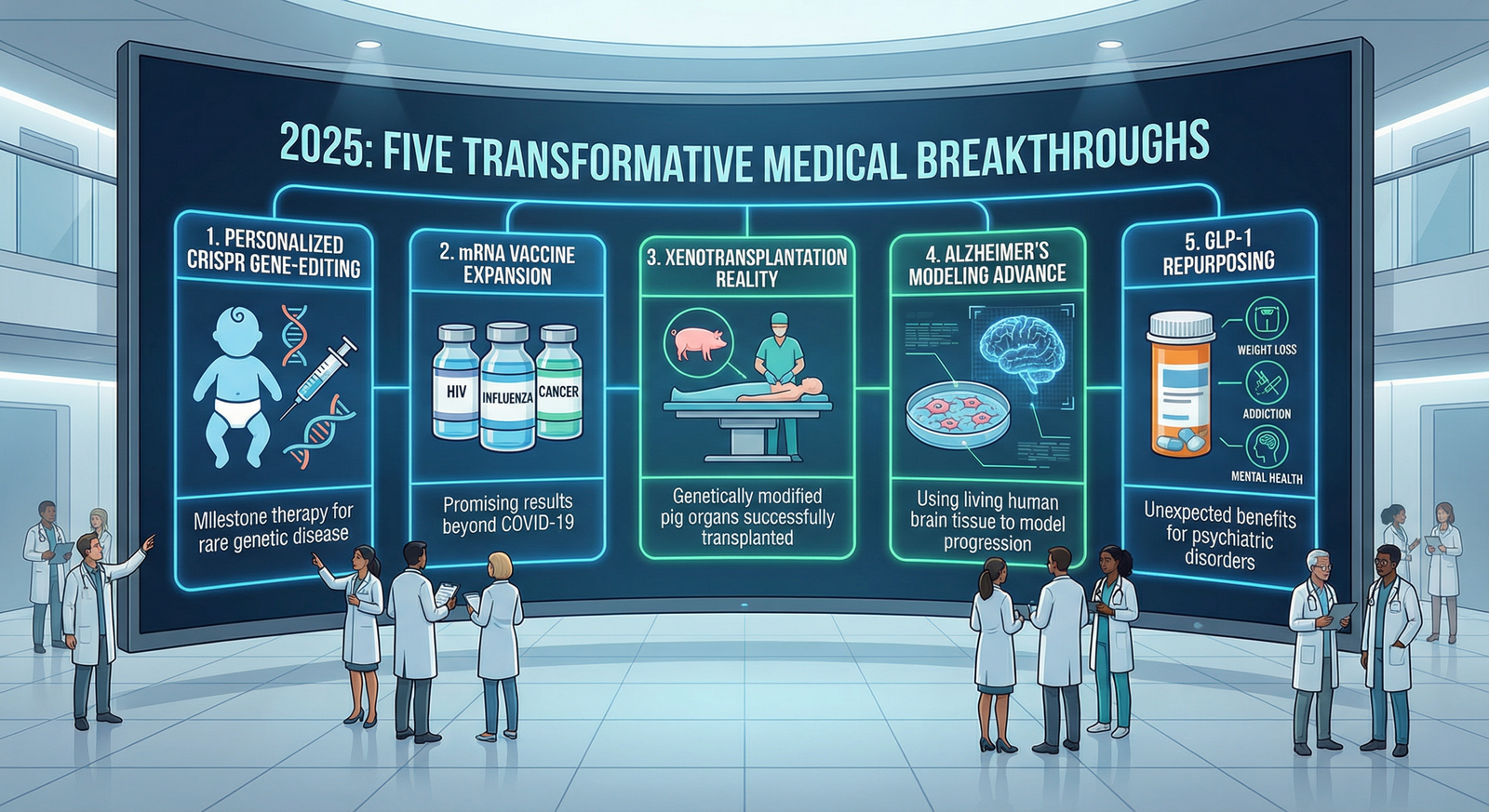
Euronews Health, in surveying leading medical journals and scientific publications, identified five medical breakthroughs that most profoundly represent reasons for hope: innovations that could eventually transform healthcare delivery for millions of people globally. These breakthroughs span genetic medicine, immunology, organ transplantation, neurodegenerative disease research, and pharmaceutical applications—representing the breadth of modern medicine's innovative capacity.
Breakthrough #1: CRISPR Gene Editing Treats a Baby's Rare Genetic Disease
CRISPR in Medicine - Innovative Genomics Institute (IGI)
A Historic First: Personalized Gene Therapy in Living Patients
In February 2025, the world witnessed a historic medical milestone: a baby became the first patient to receive personalized CRISPR gene-editing therapy, with scientists directly editing faulty genes in the infant's liver to treat a rare, often-fatal genetic disorder.
This procedure represents not merely an incremental advance but rather a fundamental paradigm shift in how medicine addresses genetic disease. Rather than attempting to compensate for genetic defects through medication, enzyme replacement, or other indirect approaches, CRISPR enables direct correction of the underlying genetic causation—addressing disease at its fundamental molecular source.
The Scientific Achievement: Direct Gene Editing
The CRISPR-Cas9 System: CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) combined with the Cas9 protein functions as a molecular scissors capable of precisely cutting DNA at targeted locations. Paired with DNA repair machinery that can insert corrected genetic sequences, CRISPR enables targeted correction of disease-causing mutations.
In Vivo Editing: Historically, gene-editing therapies were performed ex vivo—extracting cells from patients, editing them in laboratory settings, and reinfusing edited cells. This approach limited application to blood-derived cells. The breakthrough in this case involves in vivo editing—directly editing genes within the patient's body (specifically liver tissue), representing far greater technical complexity but vastly broader therapeutic potential.
Personalized Approach: Rather than developing a standardized treatment for a disease affecting many patients, this therapy was customized to this particular infant's specific genetic mutation—representing a new paradigm of truly personalized medicine.
Clinical Outcomes and Implications
Immediate Benefits: Following gene-editing treatment, the baby's medication dependence for managing the genetic disorder dramatically reduced, and quality of life substantially improved. By November 2025, the patient was achieving developmental milestones including walking—remarkable progress for a child with a previously devastating, often-fatal condition.
Long-Term Monitoring: While immediate results are encouraging, the patient requires lifelong monitoring to assess treatment durability, potential off-target genetic effects, and long-term safety. This ongoing surveillance represents standard practice for novel genetic therapies.
Broader Implications: Researchers emphasize this procedure's success indicates that other patients with various genetic disorders may benefit from CRISPR-based therapy. The range of potentially treatable conditions extends to inherited metabolic disorders, hemophilias, thalassemia, sickle cell disease, and numerous other monogenic (single-gene) genetic conditions.
Despite this breakthrough, substantial barriers to widespread CRISPR application persist: manufacturing complexity, cost, delivery methodology to reach target tissues, potential off-target editing effects, and regulatory approval timelines. These challenges mean CRISPR therapies will initially remain limited to severe conditions lacking alternative treatments, with broader application developing over years.
Breakthrough #2: mRNA Vaccine Technology Expands Beyond COVID-19
mRNA vaccines for infectious diseases: principles, delivery ...
The Evolution of a Revolutionary Platform
The COVID-19 pandemic accelerated development of mRNA vaccine technology—utilizing messenger RNA encoding viral proteins rather than traditional approaches using weakened/inactivated virus, viral vectors, or protein subunits. While mRNA vaccines gained prominence during the pandemic, their theoretical potential extends far beyond infectious disease prevention.
In 2025, this potential began translating into clinical reality, with hundreds of clinical trials evaluating mRNA-based vaccines for influenza, HIV, malaria, genetic diseases, and cancer—representing unprecedented diversity in therapeutic application.
Promising 2025 Results: HIV and Cancer Vaccines
HIV Vaccine Advances: Multiple studies published in summer 2025 demonstrated that mRNA-based HIV vaccines can induce neutralizing antibodies—antibodies capable of recognizing and inactivating diverse HIV variants, representing the immunologic basis for effective vaccine protection. This achievement addresses a decades-long challenge: HIV's extreme genetic variability has historically defeated vaccine development efforts. mRNA's flexibility in encoding various HIV proteins simultaneously offers theoretical advantage in generating broadly neutralizing immune responses.
Cancer Vaccine Development: Beyond infectious disease prevention, mRNA technology is being applied to personalized cancer vaccines. These vaccines encode mutations specific to individual tumors, theoretically training immune systems to recognize and destroy cancer cells while sparing normal tissue. Multiple early-stage trials are generating encouraging results.
Other Infectious Disease Applications: Trials are ongoing for influenza, respiratory syncytial virus (RSV), malaria, and numerous other infectious diseases, with several showing promising immunogenicity data.
Mechanistic Advantages of mRNA Vaccines
Several characteristics make mRNA vaccines particularly attractive:
Rapid Development: Unlike traditional vaccines requiring months or years for development, mRNA vaccines can be designed and manufactured in weeks—critical advantage for pandemic response or personalized cancer vaccines.
Flexibility: The same manufacturing platform can be applied to any target antigen—whether viral protein, cancer mutation, or genetic disease protein—by simply changing the mRNA sequence.
Strong Immune Response: mRNA vaccines trigger robust both cellular (CD8+ T-cell) and humoral (antibody) immune responses, more comprehensive than many traditional vaccine approaches.
Safety Profile: Unlike viral vector vaccines, mRNA vaccines cannot integrate into host genome, eliminating concerns about insertional mutagenesis. mRNA is rapidly degraded, reducing long-term exposure.
Realistic Assessment: Ongoing Challenges
While 2025 results are promising, researchers emphasize that larger studies with more participants are required to understand the full potential and limitations of mRNA vaccination across diverse diseases. Manufacturing scale-up, storage/stability considerations, and optimal dosing schedules remain areas requiring further optimization.
Breakthrough #3: Pig Organ Transplants Move Toward Clinical Reality
Frontiers | Xenotransplantation and interspecies ...
Xenotransplantation: Solving the Organ Shortage
The global shortage of human organs for transplantation represents a profound healthcare crisis. Approximately 100,000+ patients worldwide await organ transplantation, while only ~40,000 transplants occur annually, leaving tens of thousands dying while waiting for donor organs. Xenotransplantation—transplanting organs from other species into humans—represents a potential solution to this catastrophic shortage.
In 2025, this possibility moved dramatically closer to reality.
The Landmark Achievement: First Pig Liver in a Living Human
The Patient: A 71-year-old man with advanced liver disease caused by hepatitis B and liver cancer who was ineligible for traditional human liver transplantation or conventional surgery due to his medical complexity.
The Procedure: Scientists transplanted a genetically modified pig liver into this living human patient. The modifications involved multiple genetic changes in the pig: deletion of porcine genes that would trigger human immune rejection, and incorporation of human genes that would promote transplant acceptance.
The Outcome: Remarkably, the patient survived for 171 days with the pig liver functioning, demonstrating that genetically modified pig organs can perform essential functions in human recipients. While 171 days may seem brief, it represents extraordinary progress—the first human to survive any extended duration with a xenograft.
Additional Xenotransplantation Milestones
Beyond the liver transplant, 2025 witnessed successful pig organ transplantations of:
Pig Kidneys: Multiple patients received genetically modified pig kidneys, with some surviving for weeks to months—addressing the most common transplant type (kidney transplantation).
Pig Lungs: Attempted lung xenotransplants, traditionally the most immunologically challenging, showed encouraging early results.
Pig Hearts: Following earlier dramatic attempts (including the highly-publicized David Bennett case), refined genetically modified pig hearts showed improved compatibility in 2025 cases.
Scientific Principles: Genetic Modifications
The success of xenotransplantation required multiple genetic innovations in donor pigs:
Immunologic Compatibility: Pigs naturally express proteins that trigger human complement activation and NK cell-mediated cytotoxicity, causing hyperacute rejection. Scientists deleted these porcine genes (GGTA1, CTLA4Ig, and others) to prevent immunologic attack.
Human Transgenes: Incorporation of human immune-regulatory genes (CD55, CD46, CD39) further promotes immune tolerance.
Metabolic Optimization: Genetic modifications optimize organ size, growth characteristics, and metabolic rates for human recipients.
Timeline to Clinical Availability
While 2025 achievements are remarkable, xenotransplantation remains in early clinical stages. Regulatory pathways, safety monitoring, immunosuppression protocols, and refinement of genetic modifications will require years of additional development before xenotransplantation becomes a widely available therapeutic option. However, the trajectory suggests that by 2030s, genetically modified pig organs could become standard therapeutic option for patients with end-stage organ failure.
Breakthrough #4: Living Human Brain Tissue Models Dementia Progression
The Amyloid-β Pathway in Alzheimer's Disease | Molecular ...
A World-First Achievement in Neuroscience
In a world-first development, scientists in the United Kingdom achieved direct study of early dementia progression using living human brain tissue. This breakthrough addresses one of medicine's most vexing challenges: understanding and treating Alzheimer's disease and other dementias despite absent effective therapies.
Source Tissue: Rather than relying on animal models or computational simulations, researchers obtained healthy human brain tissue from patients undergoing unrelated surgeries—utilizing tissue that would otherwise be discarded.
Experimental Exposure: This living human brain tissue was exposed to amyloid-beta (Aβ), a toxic protein implicated in Alzheimer's disease pathogenesis. Amyloid-beta accumulates abnormally in Alzheimer's brains, forming plaques that researchers hypothesize contribute to neurodegeneration.
Real-Time Observation: Using advanced microscopy and recording techniques, scientists observed in real time how amyloid-beta exposure caused destruction of connections between brain cells (synaptic loss)—the cellular basis of cognitive decline in Alzheimer's disease.
Why This Matters: The Alzheimer's Disease Challenge
Alzheimer's disease represents one of medicine's greatest unsolved problems:
Burden and Prevalence: Approximately 50+ million people worldwide have dementia (70% Alzheimer's disease), increasing to 130+ million by 2050 as populations age. The personal, family, and societal burden is immeasurable.
Absence of Effective Treatment: Despite intensive research for decades, no disease-modifying treatment currently exists. Available medications provide only modest symptomatic benefits.
Animal Model Limitations: Historically, Alzheimer's research relied on transgenic mice engineered to overexpress Alzheimer's-associated proteins. However, mice don't naturally develop Alzheimer's disease, and therapeutic advances in mice frequently fail in human trials—a persistent source of frustration.
Amyloid Hypothesis Debate: While amyloid-beta accumulation is central to Alzheimer's pathology, recent evidence suggests amyloid alone may not be sufficient to cause cognitive decline—other factors (tau tangles, neuroinflammation, vascular dysfunction, metabolic dysfunction) play important roles.
Research Impact: Accelerating Drug Discovery
Direct study of how pathogenic processes occur in actual human brain tissue offers several advantages:
Disease Relevance: Findings in human tissue are far more likely to translate to human disease than findings in animal models.
Mechanism Discovery: Real-time observation of how amyloid-beta causes synaptic loss may reveal therapeutic targets not apparent in animal studies.
Drug Screening: This human tissue system could enable rapid screening of candidate Alzheimer's treatments to identify those most likely to prevent synaptic loss.
Personalization: Eventually, patient-derived brain tissue could enable testing of treatments tailored to individual patients' specific disease mechanisms.
Realistic Timeline: Long Path Ahead
While this breakthrough is significant, development of new Alzheimer's treatments requires years of further research, animal studies, and clinical trials. A treatment discovered from this platform in 2025 would likely require 10-15+ years of additional development before becoming available to patients. However, this work significantly accelerates the pathway.
Breakthrough #5: Weight Loss Drugs Show Unexpected Broader Benefits
GLP-1 Medications for Weight Loss: Semaglutide vs ...
From Weight Loss to Psychiatric and Addiction Medicine
A surprising breakthrough emerged in 2025 as researchers discovered that blockbuster GLP-1 receptor agonist medications—originally developed for diabetes and obesity—may also treat addiction and psychiatric disorders including schizophrenia. This unexpected efficacy suggests GLP-1 medications may represent far broader therapeutic tools than initially anticipated.
The Medications: GLP-1 Receptor Agonists
Background: GLP-1 (glucagon-like peptide-1) receptor agonists include semaglutide (Ozempic, Wegovy), tirzepatide, and others. These medications were originally developed for type 2 diabetes management, where they improve blood glucose control.
Weight Loss Efficacy: In recent years, these agents proved dramatically effective for weight loss even in non-diabetic patients, with studies demonstrating 15-20% body weight reduction—far superior to previous weight loss medications.
Mechanism: GLP-1 agonists act on multiple physiologic systems: enhancing insulin secretion, slowing gastric emptying (promoting satiety), activating brainstem appetite centers, and potentially improving glucose sensing.
2025 Studies: Psychiatric and Addiction Applications
Addiction Treatment: Multiple 2025 studies examined whether GLP-1 agonists reduce substance use and addiction behavior. Preliminary results suggest potential benefits for alcohol, opioid, and other substance addictions.
Schizophrenia and Psychotic Disorders: Studies found that weight loss medications may improve symptoms in patients with schizophrenia—particularly negative symptoms (social withdrawal, emotional blunting) and cognitive impairment.
Proposed Mechanisms: The mechanisms underlying these psychiatric effects likely involve:
· Weight Loss Benefit: Obesity itself is associated with depression, anxiety, and cognitive impairment—weight loss alone may improve psychiatric symptoms
· Improved Vascular Function: GLP-1 agonists improve endothelial function and blood flow—potentially enhancing cerebral perfusion and brain metabolic function
· Anti-inflammatory Effects: These medications reduce systemic inflammation implicated in depression and psychosis
· Reward Circuit Modulation: Direct GLP-1 receptor expression in reward-processing brain regions may affect dopamine signaling relevant to addiction and psychotic symptoms
Important Caveats and Realistic Assessment
Despite promising preliminary results, researchers emphasize critical limitations:
No Cognitive Benefit in Dementia: In November 2025, Novo Nordisk (manufacturer of semaglutide) announced that studies showed semaglutide had no effect on cognition and functioning in patients with dementia or mild cognitive impairment—indicating these medications are not universal cognitive enhancers.
Not Cure-Alls: While results are encouraging for addiction and certain psychiatric conditions, GLP-1 agonists are not cure-alls. Response rates remain variable, and benefits in some conditions are modest.
Long-Term Durability Unknown: Most studies are relatively short-term. Whether benefits persist with years of treatment requires further evaluation.
Side Effects and Tolerability: GLP-1 agonists carry side effects including gastrointestinal symptoms (nausea, constipation, diarrhea), injection site reactions, and rare but serious concerns (pancreatitis, thyroid effects).
Population Heterogeneity: Response varies substantially between individuals—some experience dramatic improvement while others show minimal benefit.
Broader Context: Expanding Drug Indications
The discovery of psychiatric benefits from obesity medications represents an example of drug repurposing—identifying additional therapeutic uses for existing medications. This approach has historically accelerated drug development, provided cost-effective treatment access, and sometimes revealed important connections between disease mechanisms (in this case, suggesting shared pathophysiology linking obesity, addiction, and psychosis).
Collective Impact: Why These Five Breakthroughs Matter
Frontiers | Advances in mRNA Vaccines for Infectious Diseases
Transforming Medicine's Fundamental Approach
Individually, each breakthrough represents significant medical progress. Collectively, they suggest medicine is fundamentally evolving in several ways:
Molecular Precision: Rather than treating symptoms or disease categories broadly, modern medicine increasingly targets specific molecular drivers. CRISPR directly corrects genetic mutations; mRNA vaccines target specific antigens; GLP-1 agonists modulate specific receptor systems.
Personalization: Whether through personalized CRISPR therapy or tumor-specific cancer vaccines, medicine is moving away from one-size-fits-all approaches toward individualized treatment.
Multi-System Recognition: GLP-1 medications demonstrating benefits across obesity, diabetes, addiction, and psychiatric conditions highlight interconnections between physiologic systems that traditional medical silos overlooked.
Translational Speed: The acceleration from fundamental discovery to clinical application (as exemplified by mRNA vaccines transitioning from COVID to HIV vaccines within 3 years) represents unprecedented pace.
Global Healthcare Implications
These breakthroughs carry profound implications for global healthcare equity:
Disease Access: As xenotransplantation becomes clinically available, organ shortage—which currently condemns tens of thousands annually—could be transformed.
Developing World Impact: mRNA vaccine technology enables rapid response to emerging infectious disease threats, particularly relevant to lower-income countries with limited healthcare infrastructure.
Neurodegeneration Treatment: Dementia research advances are critical for aging populations worldwide, where dementia represents one of the primary health challenges.
Precision Medicine Democratization: As CRISPR and gene therapies advance, reducing costs will be essential to ensuring equitable access rather than benefit accruing exclusively to wealthy populations.
Challenges Ahead: Realistic Perspective
Dementia linked to diabetes, herpes as research looks beyond ...
While 2025 breakthroughs provide genuine hope, substantial challenges and uncertainties remain:
Moving from research successes to widespread clinical availability requires tremendous manufacturing expertise and investment. CRISPR therapy manufacturing currently involves complex protocols and high costs. mRNA vaccine manufacturing at global scale presents logistical challenges. Xenotransplant organ procurement and standardization requires agricultural and biotechnological infrastructure.
Regulatory and Ethical Frameworks
Gene therapies, particularly when involving modifications to heritable cells, raise profound ethical questions. Regulatory pathways must balance rapid patient access against ensuring long-term safety. International consensus on xenotransplantation regulations remains evolving.
Early adoption of breakthrough therapies typically occurs in wealthy healthcare systems. Ensuring that CRISPR gene therapy, mRNA vaccines, and xenotransplantation benefit patients globally rather than creating a two-tiered healthcare system divided by economics remains a critical challenge.
Revolutionary therapies like CRISPR require lifelong safety monitoring to identify delayed adverse effects. This long-term surveillance infrastructure must be established globally.
Conclusion: Reason for Hope Tempered With Realistic Optimism
The five medical breakthroughs of 2025—CRISPR gene therapy correcting genetic disease, mRNA vaccines expanding beyond COVID, xenotransplantation solving organ shortage, living human brain tissue revealing dementia mechanisms, and GLP-1 medications demonstrating unexpected psychiatric benefits—collectively provide genuine reason for hope regarding medicine's future.
These advances demonstrate that fundamental challenges long considered insurmountable now appear addressable. The future healthcare landscape will likely feature personalized genetic corrections, universal vaccine platforms preventing diverse diseases, organ availability removing transplant scarcity, and therapeutic approaches simultaneously addressing interconnected medical conditions.
Yet this optimism must remain tempered with realistic recognition that translating these breakthroughs into global clinical benefit requires not merely scientific innovation but also manufacturing advances, regulatory frameworks, healthcare infrastructure development, and most importantly, commitment to ensuring these powerful tools benefit all people rather than only wealthy populations.
As 2025 ends, these five breakthroughs represent inflection points—moments when medicine fundamentally changed trajectory from treating disease symptoms to addressing underlying causes. The outcomes of whether these breakthroughs fulfill their promise, and whether they become equitably accessible globally, will define healthcare for decades to come.








Post your opinion
No comments yet.